Table of Contents
What Is a Spectrum?
A spectrum is a rainbow! This rainbow is formed when a beam of white light is broken up into its constituent colors, as with a prism. The colors formed are ordered according to their wavelength. When scientists look at this rainbow, they measure how strong the light in each color is. Is blue brighter than yellow, or is this specific red brighter than this other red?
When a material interacts with light, the properties of that material are imprinted on the light. This stamp is like a unique fingerprint for each element and molecule. By examining the intensity of light in each color, scientists can work backwards to estimate the properties of materials such as the size, temperature, speed and composition of an object that the light touched along the way.
What Is Spectroscopy?
Spectroscopy is the study of the spectra produced when materials interact with or emit light. This is the key to reveal details that cannot be revealed through a picture. A spectrograph – sometimes called a spectroscope or spectrometer – breaks light from a material into its component colors in the same way that a prism splits white light in a rainbow. It records this spectrum, which allows scientists to analyze the light and discover the properties of the materials it interacts with. Spectroscopy is just as important as imaging to understanding the universe.
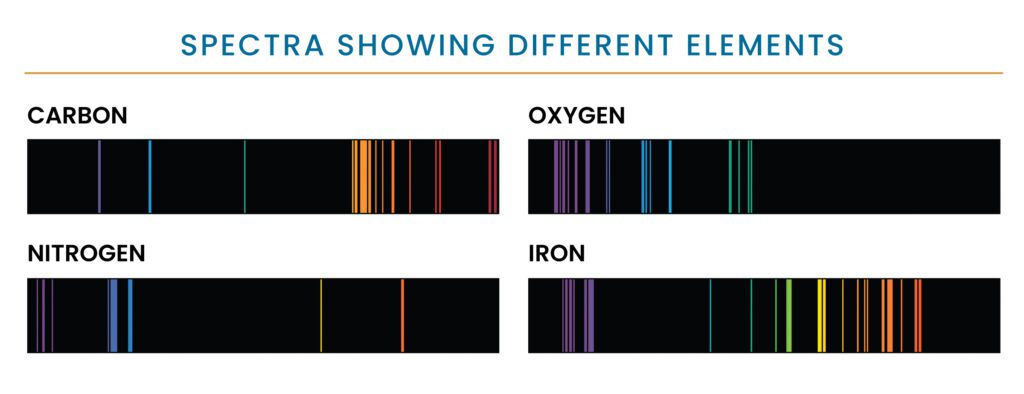
Absorption spectra of carbon, oxygen, nitrogen and iron. Each element emits a unique pattern of colors, or wavelengths, of visible light. Astronomers can analyze the patterns of light given off by an object like a star to find out what elements it is made of.
What is a Spectroscope?
A spectroscope teaches scientists about the composition of objects. So, what does a spectroscope do? A spectroscope is defined as an instrument that measures the chemical makeup of an object or the spectrum of light. Because light carries information about the materials found in objects, spectroscopes can be used to separate visible white light into a pattern of wavelengths that shows whether the object absorbs, emits, or emits light. reflects. Scientists analyze spectrum patterns to determine properties such as the intensity, frequency, and atomic composition of an object’s light.
Spectroscopes have helped astronomers obtain information about the universe. Discoveries have been made about the chemical composition of stars, planets, nebulae and galaxies. For example, the spectral pattern of a star can be compared to the spectral patterns of hydrogen, oxygen, and carbon. Astronomers may find that a star is composed of the latter atoms if they have similar absorption lines in their spectra. Therefore, astronomers can determine the overall physical and chemical state of the star. Astronomers have obtained a vast amount of information about the universe. Spectroscopes have discovered the behavior of black holes, galaxy clusters, and how the Milky Way stars continue to form.
How Does a Spectroscope Work?
A spectroscope takes incoming light and shines it through a prism. The prism refracts white light and splits it into different colors. Colored lights bounce off the beams to point in different directions. As a result, the viewer is able to see the colors in a band as absorption lines. Absorption lines are dark lines on a spectrum that can be used to determine what elements are found in an object based on the location of the absorption line. Different colors are different wavelengths of light. A wavelength is the distance between two peaks or two troughs. Wavelength is used to determine the frequency of a light wave and the distance the wave can travel. Light waves with longer wavelengths have a lower frequency and travel longer distances. Also they have less energy. Red, orange, yellow and green colors of light have longer wavelengths. Light waves with shorter wavelengths have higher frequencies and travel shorter distances. They have low energy and are blue, indigo and violet colors of light.
Spectroscopes also serve to help scientists determine the chemical composition of atoms. When atoms are excited, they emit light and this light can be focused through a spectroscope to form an atomic spectrum. An atomic spectrum can be compared to absorption lines in a spectrum. An atomic spectrum is a set of colored lines produced by a spectroscope as a result of the interactions of excited elements. Hydrogen, carbon and oxygen produce spectrum patterns that differ. The absorption lines in the atomic spectrum of a known element and the spectrum of an unknown material can be compared to determine what elements the unknown material is composed of.





How Do You Read a Spectrum?
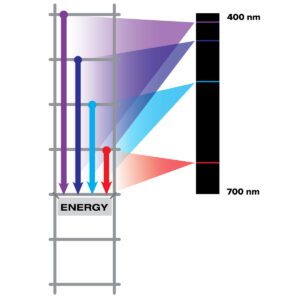
Light carries information about the material it interacts with. Different materials interact with light in different ways, and we can use light to understand what something is made of. All matter is made of atoms. Electrons move around the nucleus of an atom at different allowed energies, like rungs on a ladder. Light with the exact energy needed to go between phases can be absorbed, but not others. The electrons fall to the lower rungs, emitting light at specific energies of the gap between the rungs. This allows different atoms and molecules to emit different colors of light. The spectrum of sodium does not look like the spectrum of nitrogen – nor does the spectrum of any other element.
All elements absorb and emit specific wavelengths of light that correspond to those energy levels. An absorption spectrum is a spectrum of light transmitted through a substance, showing dark lines or bands where the light has been absorbed by the atoms, causing degradation of the spectrum. An emission spectrum is created by the electrons falling down the energy ladder. This is what you get when you look at hot gas that has been heated by something that is out of sight. This heat moves the electrons up the ladder, then when they fall down the ladder some of the light they emit comes back to you. This results in bright, colored spikes caused by atoms emitting light at those wavelengths.




Key Terms
Absorption spectrum – a record of the wavelengths (or frequencies) of electromagnetic radiation absorbed by a substance; The absorption spectrum of each pure substance is unique.
CCD Camera/Array – A charge coupled device that converts an optical signal (light) into an electrical signal for transfer to a video display or computer.
Collimator — An optical element that aligns incoming light rays so that they are parallel.
Diffraction grating – a dispersive element having a surface with very fine, closely spaced grooves, which reflect or refract (bend) different wavelengths of light by different amounts.
Emission spectrum – the colors of the light emitted by the hot gas. An emission spectrum is typically viewed through a slit, and the viewing optics produce an image of the slit in any given color of light. The emission spectrum appears as a line of colored lines, and is thus commonly called spectral lines.
Imaging spectrometer – an imaging instrument capable of determining the radiant intensity of the spectral components of a surface being imaged.
monochromator – a device that selects discrete wavelengths of light; This often includes a diffraction grating.
spectrograph – instrument for dispersing light into its spectrum of wavelengths then for photographing that spectrum.
Spectrometer – an instrument for determining the radiation intensity of atomic spectra.
Read Also
- MCQ on Chromatography
- High Performance Liquid Chromatography | HPLC
- Specific Gravity Analyzer
- What is LVDT ? | Types & Applications
- What is Rupture Disc ?