Table of Contents
What is pH ? | What is pH value ? | what is meant by pH?
pH is the formal name for the potential of hydrogen. The amount of hydrogen is given as the definition of pH, which is the negative logarithm of the concentration of H+ ions. The pH scale, which also measures how acidic or basic a solution is, describes the amount of hydrogen ions present in a solution. We know that pH measurement is important because all the acids and bases do not react with the same chemical compound at the same rate. Some react very vigorously, some moderately while others show no reaction. To determine the strength of acids and bases quantitatively, we use a universal indicator which shows different colors at different concentration of hydrogen ion in solution. Generally, the value of pH of acids and bases are used to quantitatively determine their strength.
What is ph definition?-
“pH is defined as the negative logarithm of H+ ion concentration. Hence the meaning of the name pH is justified as the power of hydrogen.”

A pH scale is a tool for measuring acids and bases. The scale ranges from 0-14. Litmus paper is an indicator used to tell if a substance is an acid or a base.
A solution with a pH less than 7 is considered acidic; a solution with a pH greater than 7 is considered basic, or alkaline.
How to Measure pH?
we can measure the pH by following method.
1. Measuring pH Using an Indicator:-
This category basically includes two methods: One involves comparing the standard color corresponding to a known pH with the color of an indicator immersed in the test liquid using buffer solution. The other method involves preparing pH test paper which is soaked in the indicator, then immersing the paper in the test liquid and comparing its color with the standard color. This method is simple, but prone to error. A high degree of accuracy cannot be expected.





The indicator method cannot measure the pH of high-purity water, since the influence of the indicator itself is too large.
2. Hydrogen-Electrode Method:
A hydrogen electrode is made by adding platinum black to platinum wire or a platinum plate. It is immersed in the test solution and an electric charge is applied to the solution and the solution is saturated with hydrogen gas. The electrode potential is measured between platinum black electrode and silver chloride electrode. This potential is inversely proportional to pH of the solution.





3. Quinhydron-Electrode Method
When quinhydrone is added to a solution, it separates into hydroquinone and quinone.
Because quinone’s solubility varies depending on the pH value of the solution, pH can be determined from the voltage between a platinum and reference electrode.
Although this method is simple, it is seldom used today, because it does not work when oxidizing or reducing substances are involved, or when the test solution has a pH above 8 or 9.
Note: Quinhydron solution of a certain pH is sometimes used to check whether an ORP meter is operating normally. The principle of the quinhydron electrode is applied in such a case.
4. Antimony-Electrode Method
This method involves immersing the tip of a polished antimony rod into a test solution, also immersing a reference electrode, and measuring pH from the difference in potential between them. This method was once widely used because the apparatus is sturdy and easy to handle. However, its application is now quite limited because results vary depending on the degree of polish of the electrode, and reproducibility is low.





Note: This method is now used only in cases where a high degree of accuracy is not required.
5. Glass-Electrode Method
The glass electrode method uses two electrodes, a glass electrode and reference electrode, to determine the pH of a solution by measuring the voltage (potential) between them.
This method is the one most commonly used for pH measurement, since the potential quickly reaches equilibrium and shows good reproducibility, and because the method can be used on various types of solutions, with oxidizing or reducing substances having very little impact on the result.
The glass electrode method is widely used, not only in industry but also in many other fields.





In its “Methods of pH Measurement” “Since measurement using a hydrogen electrode is not necessarily appropriate, measurement using a glass electrode is recommended for industrial pH measurement.”
6. Semiconductor sensor methods
The semiconductor pH sensor, whose development started around 1970, replaces a glass electrode with a semiconductor chip.





This sensor, known as an ion sensitive field effect transistor (ISFET), is not only resistant to damage but also easily miniaturized. Miniaturization allows the use of smaller amounts of sample for measurement, and makes it possible to perform measurements in very small spaces and on solid state surfaces. This sensor promises useful applications in measurement in the fields of biology and medicine.





Faqs:
what is the full form of pH ? what is the meaning of ph ?
Potential of Hydrogen is what pH is formally known as. The negative logarithm of the concentration of H+ ions is known as pH. Thus, the definition of pH as the amount of hydrogen is provided. The hydrogen ion concentration in a solution is described by the pH scale, which also serves as a gauge for the solution’s acidity or basicity.
What is pH scale ? | what is the range of the ph scale ? | what is ph level ?
The pH scale determines how acidic or basic water is. The range is 0 to 14, with 7 representing neutrality. Acidity is indicated by pH values below 7, whereas baseness is shown by pH values above 7.
What is the ph range of our body ?
The pH of your body is between 7.35 and 7.45. This implies that body is mildly basic or alkaline by nature. Your stomach acid, in contrast, has a pH range of 1.5 to 3.5.
what is the ph of blood ? what is the ph value of blood
The pH range of your blood is typically 7.35 to 7.45. In other words, blood is naturally somewhat basic or alkaline.
what is the ph of water ? | what is the ph value of water ? what is the ph of pure water ?
Water has a pH of 7, which is exactly in the middle of the pH scale, in its purest state. The pH of water can be altered by particles, and the majority of usable water has a pH of between 6.5 and 8.5.
what is ph meter ?
A pH metre is a device that measures a solution’s acidity or alkalinity. pH is a unit of measurement for the degree of acidity or alkalinity. It is rated from 0 to 14 on a scale.
What is soil ph ?
Any pH result less 7 indicates acidity, and any pH over 7 indicates alkalinity. A neutral soil has a pH of seven. Because it affects how readily available vital nutrients are, pH is significant. In soils with a pH between 6 (slightly acidic) to 7.5(slightly alkaline), the majority of horticultural crops will grow successfully.
what is the ph of acid rain ?
The pH of typical, pure rain ranges from 5.0 to 5.5, which is somewhat acidic. However, rain becomes significantly more acidic when it interacts with sulphur dioxide or nitrogen oxides, which are produced by power plants and automobiles. Acid rain typically has a pH of 4.0.
what is the ph of distilled water ?
Immediately following distillation, the pH of distilled water is 7, but a few hours later, it has absorbed carbon dioxide from the environment and has a pH of 5.8, making it acidic.
who discovered ph scale ? | who invented ph scale | who introduced ph scale ?
Danish chemist Peter Lauritz Sorensen first proposed the idea of pH in 1909 as a practical way to express acidity.
how to calculate ph ?
You need to know the hydronium ion concentration in moles per litre to determine the pH of an aqueous solution (molarity). The equation pH = – log [H3O+] is then used to determine the pH.
How to calibrate ph meter ?
When calibrating a pH metre, measuring substances with known pH values are used. These substances are referred to as buffers, and the pH measurements on the pH metre are set to those values. When measuring additional chemicals, the pH metre refers to the calibration measurements as a reference.
what is the ph of saliva
The pH of saliva typically ranges from 6.2 to 7.6, with 6.7 serving as the average, and the pH of the mouth at rest never drops below 6.3. Saliva helps to keep the pH of the mouth cavity close to neutral (6.7–7.3).
what is the ph of urine
Between 4.5 and 8, the pH of urine is considered normal.
what is ph paper
Using a pH paper, you can determine if a solution is basic, acidic, or neutral. To do this, dip a portion of the paper into the solution, and then note how the colour changes. Due to the chemical flavin, pH paper changes colour in various solutions.
what is the ph of lemon juice
The pH range of lemon juice is 2 to 3.
what is the ph of milk
Milk is an acid-forming food, whether it is pasteurised, canned, or dried. With a pH between 6.7 and 6.9, it is acidic.
what is the ph value of sodium chloride
Since sodium chloride is a neutral substance, its pH value is seven.
are plants and animals ph sensitive
All living things are sensitive to pH, which is why our bodies only function properly between the limited pH ranges of 7.0 and 7.8.
what is the ph of gastric juice released during digestion
Additionally, the stomach releases hydrochloric acid, which has a pH lower than 7, to create an acidic environment. The gastric juices in the stomach have a pH in the range of 2 to 5, which means they have a mildly acidic composition.
what is the ph of milk of magnesia | what is the ph value of milk of magnesia
The pH of milk of magnesia, an aqueous suspension of solid magnesium hydroxide, is 10.52.
what is the ph value of neutral solution
Neutral solution, which has a pH of 7.
what is the ph value of saliva after meal
Before eating, saliva has a pH of around 7. After eating, saliva has a pH of about 5.
ph of vinegar
With a pH of 2-3, vinegar has a mild acidic nature.
ph test strips
Litmus paper is used to create PH test strips. To evaluate if a product or liquid is acidic, basic, or neutral in nature, PH test strips are utilised.
telegra ph,
With the help of the straightforward publishing tool Telegra.ph, you can quickly compose posts with complex formatting and publish them online.
Read Also:-
Related Search:-





I to H Converter
I to H Converter





Paddle level switch working principle
Paddle level switch working principle
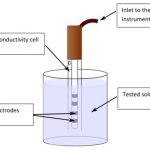




How to Measure Conductivity?
How to Measure Conductivity?
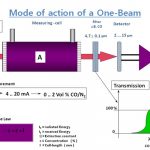




How Infra Red(IR) Gas analyzer Work
How Infra Red(IR) Gas analyzer Work





Interview Questions
Interview Questions




Actuators
Actuators