Table of Contents
Conductivity measurement is an extremely widespread and useful method, especially for quality control purposes so it is important to know How to measure conductivity?
What is conductivity?
Conductivity is the ability of a solution, a metal or a gas – in brief all materials – to pass an electric current. In solutions the current is carried by cations and anions whereas in metals it is carried by electrons. How well a solution conducts electricity depends on a number of factors:
- Concentration
- Mobility of ions
- Valence of ions
- Temperature
All substances possess some degree of conductivity. In aqueous solutions the level of ionic strength varies from the low conductivity of ultra pure water to the high conductivity of concentrated chemical samples.

What is the Thermal Conductivity ?
Thermal conductivity is the flow of energy caused by the random movement of molecules across a temperature gradient. It can be described as a way to quantify how well a material conducts heat. K represents it. Thermal conductivity is the inverse of thermal resistivity.
Each material has a unique ability to conduct heat. The following formula describes a material’s thermal conductivity:
K = (QL)/(AΔT)
Where,
K is Thermal conductivity, measured in W/m.K
Q is the heat transmission rate (in Joules/second or Watts) through the material.
Between the two isothermal planes, L is the distance.
A represents the surface’s area in square metres.
T is the Kelvin difference in temperature
SI unit of thermal conductivity
Temperature, Length, Mass, and Time are the four dimensions used to express thermal conductivity.
Watts per meter-Kelvin or Wm-1K-1 is the SI unit for this value.
Typically, it is written as power/(length * temperature).
For every Kelvin of temperature difference, the rate of heat conduction through a material of same thickness is described by these units.
Importance of Conductivity measurement
Conductivity measurement is an extremely widespread and useful method, especially for quality control purposes. Surveillance of feed water purity, control of drinking water and process water quality, estimation of the total number of ions in a solution or direct measurement of components in process solutions can all be performed using conductivity measurements. The high reliability, sensitivity and relatively low cost of conductivity instrumentation makes it a potential primary parameter of any good monitoring program. Some applications are measured in units of resistivity, the inverse of conductivity. Other applications require the measurement of total dissolved solids (TDS), which is related to conductivity by a factor dependent upon the level and type of ions present. Conductivity measurements cover a wide range of solution conductivity from pure water at less than 1×10-7 S/cm to values of greater than 1 S/cm for concentrated solutions. In general, the measurement of conductivity is a rapid and inexpensive way of determining the ionic strength of a solution. However, it is a nonspecific technique, unable to distinguish between different types of ions, giving instead a reading that is proportional to the combined effect of all the ions present.
Conductivity measurement:-
There are two technical approaches to measure conductivity – inductive and contacting.
Inductive Conductivity Measurement:





Inductive sensors are using two electromagnetic coils usually encased in a polymer ring. An alternating voltage is applied to the driving coil, which induces a voltage in the receiving coil.
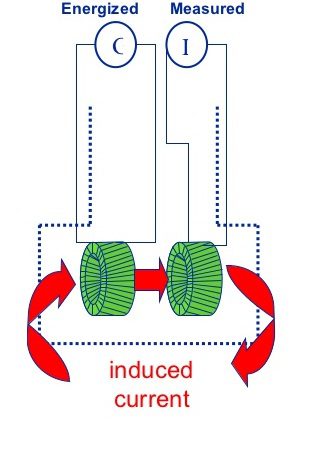




The induced current is influenced by the conductance of the solution. Due to the sealed casing, inductive sensors can be used in aggressive environments, but they are not suited for conductivities below 15 µS/cm
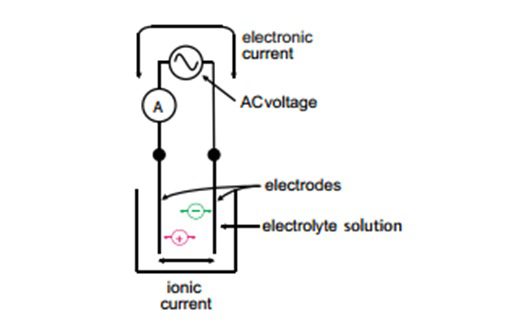
Contacting Conductivity Measurement:-
Contacting sensors consist of conducting electrodes being in direct contact with the medium. The primary parameters measured are voltage and the applied current. The interdependence of both parameters, described by Ohm’s law, allows for the calculation of a resistance R (measured in Ohm [Ω]) or the inverse – the conductance G (measured in Siemens [S]).





The contacting-type conductivity sensor usually consists of two electrodes, insulated from one another. The electrodes, typically 316 stainless steel, titanium-palladium alloy or graphite, are specifically sized and spaced to provide a known “cell constant.” Theoretically, a cell constant of 1.0 describes two electrodes, each being one square centimetre in area and spaced one centimetre apart.




Faqs:
Why does the conductivity of a solution decrease with dilution?
On dilution as solution volume rises. As a result, conductivity also falls due to a drop in the number of ions per milliliter. For a mole of ions, molar conductivity is defined. Thus, as a solution is diluted, ions become more dispersed and mobile, increasing the molar conductivity of the solution.
What is molar conductivity?
The ionic strength of a solution or the salt concentration can affect a solution’s molar conductivity. Molar conductivity is the conductance characteristic of a solution containing one mole of the electrolyte. Therefore, it is not a constant.
In other words, molar conductivity is also the ability of all the ions created by the dissolution of a mole of electrolyte in a solution to conduct electricity. The characteristic of an electrolyte solution known as molar conductivity is primarily used to assess how effectively a specific electrolyte conducts electricity in a solution. Therefore, it is not a constant.
What is limiting molar conductivity ? | what is meant by limiting molar conductivity
Limiting molar conductivity is the molar conductivity of a solution at infinite dilution. In other words, the molar conductivity is known as limiting molar conductivity as the concentration of the electrolyte approaches zero.
What is Electrical Conductivity?
Electrical conductivity is a measurement of a material’s capacity to pass an electric current. Depending on how easily electricity may pass through a substance, it has a different electrical conductivity. The material’s neutrons, electrons, and protons transport the current. Everywhere an electron goes, it carries a negative charge in contrast to the positive charge carried by protons. Electric current is the term used to describe the movement of electrons within a substance.
The Greek letter ρ stands for electrical conductivity.
The electrical conductivity, which is the inverse of resistance
σ = 1/ρ
Where,
σ is the Conductivity of electricity
ρ is resistance.
What is conductivity in water ?
A solution’s conductivity is a gauge of how well it can carry heat, sound, and electricity. Water conductivity is measured in milliohms per centimetre in CGS and Siemens per metre in SI. Water conductivity is represented by the letters k or s. Pure water serves as an insulator rather than a good conductor of electricity. If the concentration of ions in water rises, conductivity will also. Electricity can flow through distilled water when the air’s carbon dioxide levels are balanced. The ability of a solution to convey current is measured by its ionic activity, and water has a high electrical conductivity.
What is the unit of conductivity ?
Electrical conductivity is measured in siemens per metre (S/m), a SI unit.
Which has maximum conductivity?
Silver has the highest electrical conductivity. In actuality, silver is the benchmark metal against which all other metals are measured for conductivity. On a scale of 0 to 100, silver scores a perfect 100, followed by copper at 97 and gold at 76.
What does the conductivity of metals depend upon ?
The capacity of a material to conduct electricity is known as conductivity. It is depend on the mobility, valence, temperature, and accessible charge carriers (ionic).
What is specific conductivity ?
Specific conductivity is the opposite of resistivity. It is described as the conductance between the opposing faces of a conductor cube measuring one centimetre in size. The symbol for it is k. (kappa). The ability of a material to conduct electricity is measured by its specific conductivity.
How electrical conductivity of semiconductors vary with temperature? | how does conductivity of a semiconductor change with temperature ?
Because more electrons from the valence bond in semiconductors can jump to the conduction band when temperature rises, the electrical conductivity of semiconductors increases.
What is conductivity cell ?
Conductivity cells, also known as conductivity electrodes or probes, are made of a glass or plastic body with a predetermined distance between metal electrodes, encased in an outer tube. Cell constant is defined as the distance between electrodes divided by their surface area.
What is the unit of thermal conductivity ? | what is the si unit of thermal conductivity ?
Watts per metre per Kelvin, or Wm-1K-1, is the thermal conductivity unit used in the SI.
How does molar conductivity vary with concentration for weak electrolyte ?
A strong or weak electrolyte’s molar conductivity rises with dilution. As the volume of the solution grows, dilution occurs. As a result, conductivity diminishes along with the amount of ions per millilitre.
What is the unit of molar conductivity?
The conductance of a volume of electrolytic solution containing a unit mole of electrolyte put between two electrodes with unit area cross-sections or at a distance of one centimetre is generally referred to as the molar conductivity of an electrolytic solution. S⋅m2⋅mol–1 is the SI unit for molar conductivity.
Which materials are used for coating a conductivity cell ?
Depending on the application, the coating may be a conventional coating with a conductive filler like graphite, nickel, copper, silver, or gold added. Conductivity has lately been added to coatings through the use of fillers like graphene and carbon nanotubes.
What is equivalent conductivity ?
Equivalent conductivity refers to the conducting ability of all the ions produced when one gramme equivalent of an electrolyte is dissolved in a solution.
What is the value of electrical conductivity of aluminium conductor ?
Another metal with a high electrical conductivity is aluminium. Although aluminum’s conductivity is just 60% that of copper by volume, one pound of aluminium can transport two pounds’ worth of electrical current. Conductivity of aluminium is 3.5×10⁷ S/m.
Read Also:-
Related Search:-





Ultrasonic Level Switch
Ultrasonic Level Switch





Humidity Measurement
Humidity Measurement





I to H Converter
I to H Converter





Conductivity level switch
Conductivity level switch




Control Valve Components/ parts
Control Valve Components/ parts