Table of Contents
Column Chromatography Definition
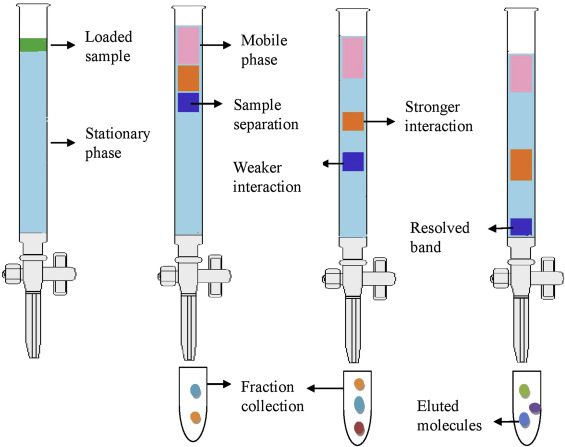
Column chromatography is used by an organic chemist to separate liquids and solids from a solution. A column of absorbent which is silica gel or it can be alumina is loaded into a column full of impurities while using it. This mixture flows down the column, and the component of the sample is separated by partitioning between the mobile eluent and the stationary packing material. In simple words, column chromatography is the separation of substances from solution in order to obtain simpler substances from complex elements. There are five types of column chromatography used in the process of separation. The process passes through two phases, that is, the mobile phase and the stationary phase.
Types of Column Chromatography
There are five types of chromatography that can be applied to the basic principles of chromatography. Gas chromatography (GC), chiral liquid chromatography (LC), ion exchange chromatography (IEC), and size exclusion chromatography (SEC), are five methods that use columns in column chromatography.
In gas chromatography, the mobile phase is a gas, and in liquid chromatography, a liquid is used as the mobile phase. Ion exchange is used in the stationary phase to ionize and separate ions and molecules. Size exclusion chromatography (SEC) and chiral chromatography are complex methods of separating molecules in a mixture that are typically used for large samples.
Principle of Column Chromatography
A sample mixture is taken, which is set on top of the column. This mixture is made to be absorbed on top of the stationary phase. The stationary phase is the next phase after the mobile phase to separate the mixture in the column. The separation of compounds is due to the polarization of the molecules, and the rate of motion moves the particles and separates them in the mixture. The polarization of the molecules in a mixture initiates the separation of the components in the mixture. After the mobile phase, the molecules are collected into small fractions in a test tube, and then separation and purification take place. By means of a rotary evaporator, the salt is separated from the mixture and the other compound is separated. The process of column chromatography is used for the separation and purification of compounds in a mixture. It is a convenient method of separation and purification of substances in a mixture widely used by a chemist. The process should have everyone’s attention and focus. You can automate the process, but the cost goes up, so the manual method is more preferred by chemists.
Application of Column Chromatography
- Column chromatography is used in the purification of compounds.
- To separate molecules from a mixture and use it to make a new substance.
- Used by a chemist to know the drug estimation in drug solution.
- It is used to separate diastereomers, to separate racemates and to separate geometrical isomers
- Used for the separation of metabolic fluid from a biological fluid.
Advantages of Column Chromatography
- The process involves separating a mixture of any
- Separation of impurities from any mixture.
- Any variety and quantity separation is possible.
- The process involves low cost and simple understanding.
- All types of solvents can be used in this process which will give the result you require.
- The process can be fully automated, which is done by large laboratories.
Disadvantages of Column Chromatography
- This is a long and time consuming process.
- Small amounts become insufficient in the separation process; Only a sufficient amount can be used for isolation.
- This process is expensive as compared to the thin paper column chromatography process.
- It is a long process, so attention and focus is required during the entire process.
- If the process is automated, the process becomes costly.
Chromatography is one of the most widely used techniques used to separate components. The mobile phase can be either a liquid or a gaseous substance. This allows the flow of molecules in the column. The mobile phase can never be solid as it does not help the components to flow through the column. However the stationary phase can be either a solid liquid or a gas. Students can now know more about Column Chromatography – Theory, Definition, Types, Applications, Advantages & Disadvantages, where they can prepare for the exam and take their revision notes.
Read Also
- What is NMR Spectroscopy?
- What is Spectroscope | Spectroscope | How to read a spectroscope
- MCQ on Chromatography
- High Performance Liquid Chromatography | HPLC