Table of Contents
- Compared with other gases, oxygen is strongly paramagnetic. The paramagnetism of oxygen may be regarded as the capability of an oxygen molecule to become a temporary magnet when placed in a magnetic field, analogous to the magnetization of a piece of soft iron. The volume magnetic susceptibility of the flowing gas sample is sensed in the detector / magnet assembly.
- As shown in the functional diagram of Figure , a dumbbell-shaped nitrogen-filled hollow glass test body is suspended on a Platinum / nickel alloy ribbon in a non-uniform magnetic field.
- Because of the“magnetic buoyancy” effect, the spheres of the test body are subjected to displacement forces, resulting in a displacement torque that is proportional to the volume magnetic susceptibility of the gas surrounding the test body.
- Measurement is accomplished by a null-balance system, where the displacement torque is opposed by an equal, but opposite, restorative torque.
- The restorative torque is due to electromagnetic forces on the spheres, resulting from a feedback current routed through a titanium wire conductor wound lengthwise around the dumbbell.
- In effect, each sphere is wound with a one-turn circular loop. The current required to restore the test body to null position is directly proportional to the original displacement torque, and is a linear function of the volume magnetic susceptibility of the sample gas.
Functional diagram Of Paramagnetic O2 Analyzer
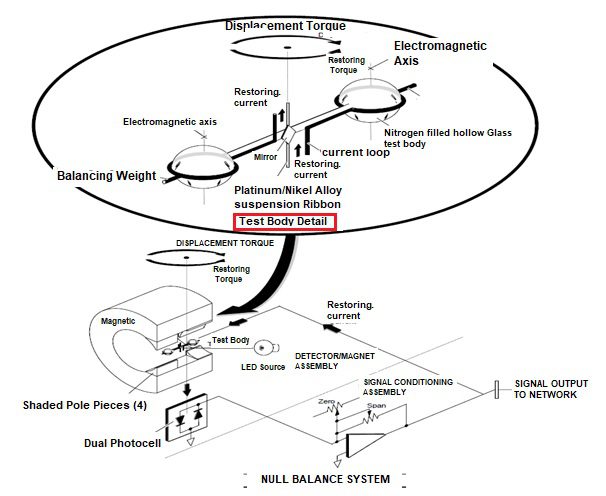
- The restoring current is automatically maintained at the correct level by an electro optical feedback system. A beam of light from the source lamp is reflected off the square mirror attached to the test body, and onto the dual photocell.
- The output current from this combination is equal to the difference between the signals developed by the two halves of the photocell. This difference, which constitutes the error signal, is applied to the input of an amplifier circuit that provides the restoring current. When the test body is in null position, both halves of the photocell are equally illuminated; the error signal is zero; and the amplifier output remains constant.
- As soon as the test body begins to rotate, however, the amounts of light become unequal resulting in application of an error signal to the input of the amplifier circuit. The resultant amplifier output signal is routed through the current loop, thus creating the electromagnetic forces required to restore the test body to null position. Additionally the output from the amplifier is conditioned to give the digital display of % of O2 and simultaneously transmits 4-20 ma output.
This analyzer is a dumbbell type Paramagnetic Type Oxygen analyzer. Because this analyzer is based on that the magnetic susceptibility of oxygen gas is larger than coexisting gases, stable measurement is ensured unaffected by coexisting gases. The detector does not have a heating part such as heater. Therefore, this analyzer is suited for measuring the oxygen concentration in flammable gas. Further, running cost can be saved since reference gas is not required. The principle of measurement is dependent on the strong magnetic property of oxygen molecules. Therefore, measurement is almost unaffected by other molecules weaker in magnetic property than oxygen. Some of its features are as follows:
- Suited for measuring oxygen in flammable gas.
- Small-sized and easy to handle.
- Usable with a wide range of power supplies.
- Output is linear
Read Also:-
How To Zirconia disc cell oxygen analyzer works
How infra Red gas analyzer work
Related Search:-





Paramagnetic Type Oxygen analyzer
Paramagnetic Type Oxygen analyzer





Air Lock Valve
Air Lock Valve





Ask Questions
Ask Questions





Pressure Transmitter Calibration
Pressure Transmitter Calibration
pH Measurement
pH Measurement