Table of Contents
Fuel cell definition-A fuel cell is an electrochemical device that converts the chemical energy of a fuel directly into electricity. Unlike traditional combustion engines, fuel cells do not burn fuel to produce energy, but instead use a chemical reaction between a fuel and an oxidant to generate electricity. Fuel cells work by combining a fuel, such as hydrogen, with an oxidizing agent, such as oxygen from the air, in the presence of an electrolyte. This chemical reaction produces electricity, water, and heat as byproducts. The most common type of fuel cell uses hydrogen as the fuel and oxygen from the air as the oxidant. When hydrogen and oxygen react in the fuel cell, they produce water, heat, and electricity. This process is clean and efficient, as the only byproducts are water and heat. Fuel cells are highly efficient, with some types achieving efficiency levels of up to 60%, compared to traditional internal combustion engines which typically have efficiencies of around 25%. Fuel cells also have the advantage of producing zero emissions when using hydrogen as the fuel, making them a promising technology for use in transportation and stationary power generation. Fuel cells are currently being used in a variety of applications, including transportation, stationary power generation, and portable electronics. However, the high cost of fuel cell systems remains a significant barrier to widespread adoption. As technology advances and costs come down, fuel cells have the potential to play a major role in our energy future.
Fuel cell diagram:-

Working principle of fuel cell | Fuel cell working:-
The working principle of a fuel cell involves the electrochemical reaction between a fuel and an oxidizing agent to produce electricity, water, and heat. The basic components of a fuel cell include the anode, cathode, and electrolyte. The fuel is typically hydrogen, which is supplied to the anode of the fuel cell. At the anode, the hydrogen molecules are split into protons (H+) and electrons (e-) in the presence of a catalyst, such as platinum. The electrons are then conducted through an external circuit to the cathode, where they recombine with the protons and the oxidizing agent (usually oxygen from the air) to form water.
Meanwhile, the protons pass through the electrolyte, which is typically a polymer membrane, to reach the cathode. The flow of protons through the electrolyte generates an electrical current that can be used to power electrical devices.
The overall reaction can be represented as:
2H2 + O2 → 2H2O
The reaction produces water as the only byproduct, making fuel cells a clean and efficient source of energy. Different types of fuel cells use different types of electrolytes and catalysts, and the specific chemical reactions and operating conditions can vary depending on the type of fuel cell. However, the basic principles of electrochemical reaction and proton exchange are the same for all types of fuel cells.
Types of fuel cell:-
There are several types of fuel cells, each with its own unique advantages and disadvantages. Here are brief descriptions of some of the most common types of fuel cells.
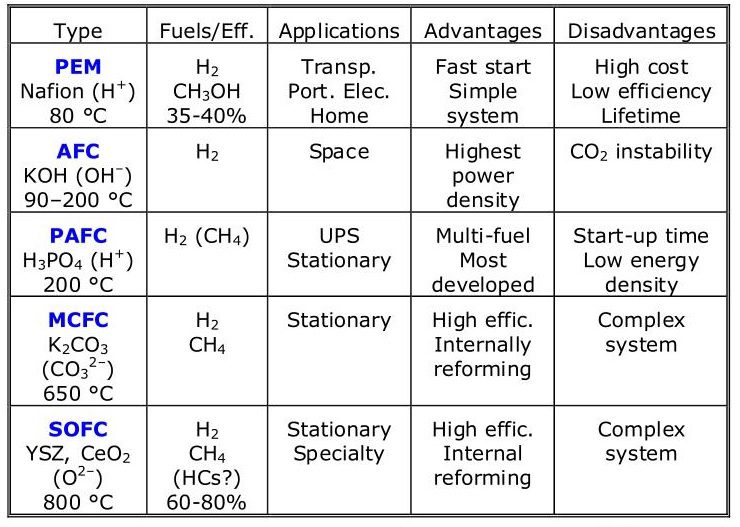
1. Proton Exchange Membrane (PEM) Fuel Cells:
PEM fuel cells use a solid polymer membrane as the electrolyte and operate at relatively low temperatures (typically between 60-90°C). They are highly efficient and can start up quickly, making them a popular choice for transportation applications such as cars and buses. 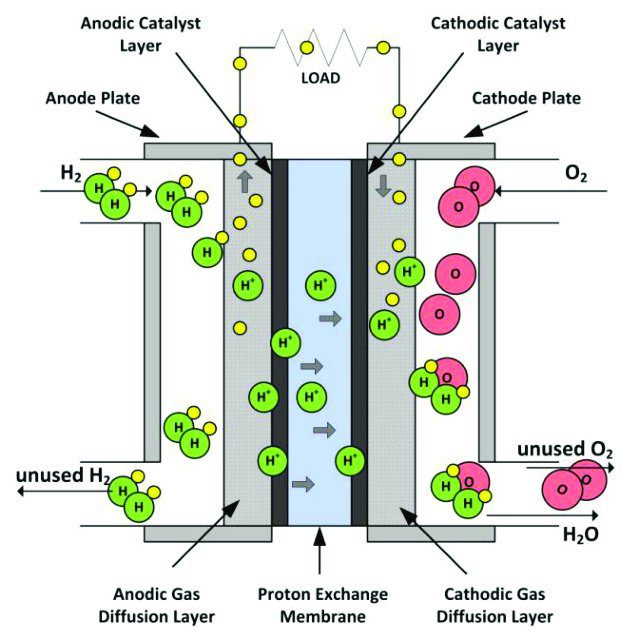




2. Solid Oxide Fuel Cells (SOFCs):
SOFCs use a solid ceramic electrolyte and operate at high temperatures (typically between 800-1000°C). They are highly efficient and can use a variety of fuels, but their high operating temperature makes them less suitable for transportation applications.




3. Alkaline Fuel Cells (AFCs):
AFCs use an alkaline electrolyte and operate at relatively low temperatures (typically between 40-80°C). They are highly efficient and can use a variety of fuels, but they are sensitive to carbon dioxide and require pure hydrogen fuel.




4. Phosphoric Acid Fuel Cells (PAFCs):
PAFCs use a phosphoric acid electrolyte and operate at temperatures between 150-200°C. They are highly efficient and can use a variety of fuels, but their high operating temperature makes them less suitable for transportation applications.




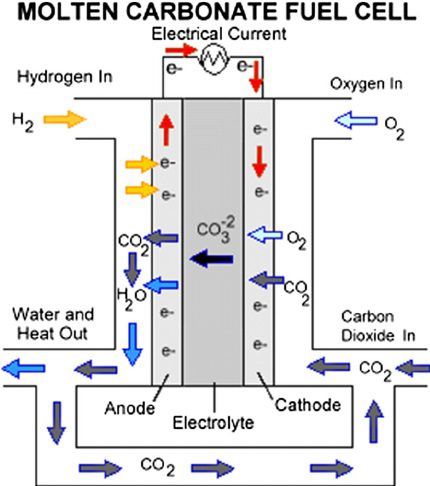
5. Molten Carbonate Fuel Cells (MCFCs):
MCFCs use a molten carbonate electrolyte and operate at high temperatures (typically between 600-700°C). They are highly efficient and can use a variety of fuels, but their high operating temperature makes them less suitable for transportation applications.




6. Direct Methanol Fuel Cells (DMFCs):
DMFCs use methanol as the fuel and operate at relatively low temperatures (typically between 50-100°C). They are highly efficient and can be used in portable applications such as laptops and mobile phones.









Fuel cell technology:-
There are still some challenges to overcome in fuel cell technology, including the cost and availability of fuel cell materials, the need for supporting infrastructure such as hydrogen refueling stations, and the need for further research and development to improve the efficiency and durability of fuel cells. Nonetheless, fuel cell technology has the potential to play an important role in our transition to a cleaner, more sustainable energy future.
Application of fuel cell
Fuel cells have a wide range of potential applications, thanks to their high efficiency, clean energy production, and versatility. Here are some of the most promising applications for fuel cells:
1. Transportation:
Fuel cells can be used to power cars, buses, trucks, and other vehicles. Fuel cell vehicles offer the potential for zero-emission transportation, with faster refueling times and longer ranges than battery-electric vehicles.
2. Stationary Power Generation:
Fuel cells can be used for stationary power generation, providing clean and reliable electricity for buildings and communities. Fuel cell power plants can be designed to run on a variety of fuels, including hydrogen, natural gas, and biogas.
3. Portable Power:
Fuel cells can be used to provide portable power for a range of applications, from mobile phones and laptops to emergency backup power for remote locations.
4. Marine Applications:
Fuel cells can be used to power boats, ships, and submarines, offering a clean and quiet alternative to traditional diesel engines.
5. Aerospace:
Fuel cells have potential applications in space exploration, as they can provide power without the need for bulky batteries or solar panels.
6. Military:
Fuel cells have potential applications in military vehicles and portable power systems, as they offer a quiet and emissions-free alternative to traditional generators and engines.
Advantages of fuel cell:-
Fuel cell technology is a promising area of research and development, with the potential to provide clean, efficient, and reliable power for a wide range of applications. Here are some of the key features and benefits of fuel cell technology:
- Efficiency: Fuel cells are highly efficient, with some types achieving efficiency levels of up to 60%, compared to traditional internal combustion engines which typically have efficiencies of around 25%.
- Clean Energy: Fuel cells produce electricity through an electrochemical process, and as long as hydrogen is used as the fuel, they produce zero greenhouse gas emissions.
- Versatility: Fuel cells can be used in a variety of applications, from portable devices such as laptops and mobile phones, to transportation applications such as cars, buses, and trains, as well as stationary power generation for buildings and communities.
- Reliability: Fuel cells can operate continuously as long as fuel and an oxidant are supplied, and they do not suffer from the same degradation issues as batteries.
- Scalability: Fuel cells can be easily scaled up or down to meet the power requirements of a given application, making them a flexible and adaptable technology.
- Safety: Fuel cells are generally considered to be safe, as they do not contain any flammable or explosive materials and can be designed with multiple layers of safety features.
Disadvantages of fuel cell:-
There are several disadvantages of fuel cell to consider, including:
- High Cost: Fuel cells can be expensive to manufacture, and the cost of the materials used in the construction of fuel cells, such as platinum, can be high. This can make fuel cell technology prohibitively expensive for some applications.
- Hydrogen Storage and Distribution: Hydrogen is a lightweight gas that requires special storage and distribution infrastructure. Currently, there are limited hydrogen refueling stations, which makes it difficult to use hydrogen fuel cells for transportation on a large scale.
- Fuel Availability: While hydrogen is the ideal fuel for fuel cells due to its clean and efficient combustion, it is not widely available, and its production is currently energy-intensive. This limits the scalability and applicability of fuel cells.
- Durability: Fuel cells are sensitive to temperature and humidity, and their lifespan can be limited by the degradation of their components, particularly the electrolyte membrane. This can reduce their reliability and increase the need for maintenance.
- Limited Applications: While fuel cells have many potential applications, they may not be the best choice for all situations. For example, in some applications, such as small portable devices, batteries may be a more cost-effective and practical choice.
- Safety Concerns: While fuel cells are generally considered to be safe, there are concerns about the potential for hydrogen leaks, which can be explosive if ignited. Additionally, the use of certain catalysts in fuel cells, such as platinum, can be toxic if not handled properly.
How does a hydrogen fuel cell work | Hydrogen fuel cell:-
A hydrogen fuel cell generates electricity through an electrochemical process that combines hydrogen and oxygen to produce water and electricity. Here is how it works:
- Hydrogen gas is supplied to the anode (negative electrode) of the fuel cell, where it is split into protons and electrons by a catalyst, typically platinum.
- The protons are conducted through a polymer electrolyte membrane to the cathode (positive electrode) of the fuel cell.
- At the cathode, oxygen from the air is supplied and combines with the protons and electrons that have traveled through an external circuit, producing water and generating an electrical current.
- The electrical current can be used to power electrical devices or stored in a battery for later use.
The overall chemical reaction can be represented as follows:
2H2 + O2 -> 2H2O + energy
The key advantage of hydrogen fuel cells is that they generate electricity with no harmful emissions other than water, making them a clean and efficient alternative to traditional combustion engines. Hydrogen fuel cells also have high energy density and can be refueled quickly, which makes them a promising technology for transportation applications, such as cars and buses.
Read Also
- Zirconia Oxygen Sensor
- TCD | Transcranial Doppler
- How to Solve On-Off Valve Problems?
- Motor Operated Valve Problems and Troubleshooting
- 100 Interview questions with answers on Control valve in simple words for competitive exams